

Lausanne, October 16, 2025 — The Lancet Haematology, a premier international hematology journal, has published the results of a groundbreaking clinical study evaluating Leman Biotech’s metabolically armored CD19 CAR-T therapy (Meta10-19) in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). This study marks a major milestone in the advancement of next-generation CAR-T therapies.
For the first time, clinical results have confirmed that metabolic enhancement through IL-10 autocrine signalling can achieve breakthrough clinical efficacy and excellent safety even at extremely low CAR-T cell doses. This challenges the conventional paradigm that “higher doses yield higher efficacy,” highlighting instead the critical importance of optimizing CAR-T cell function through metabolic reprogramming. These findings point towards a new direction for developing next-generation cell therapies.
The published article, titled “IL-10-expressing, anti-CD19 CAR T cells for patients with relapsed or refractory B-cell acute lymphoblastic leukaemia: an open-label, single-arm, phase 1 study” (DOI link:https://doi.org/10.1016/S2352-3026(25)00253-4), reports on an investigator-initiated clinical trial (NCT05747157). The trial was led by Professor Xingbing Wang of the First Affiliated Hospital of the University of Science and Technology of China (Anhui Provincial Hospital), in collaboration with teams from Zhejiang University, the Swiss Federal Institute of Technology Lausanne (EPFL), and Leman Biotech. The investigational product, Meta10-19, was designed, developed, and provided free of charge by Leman Biotech.
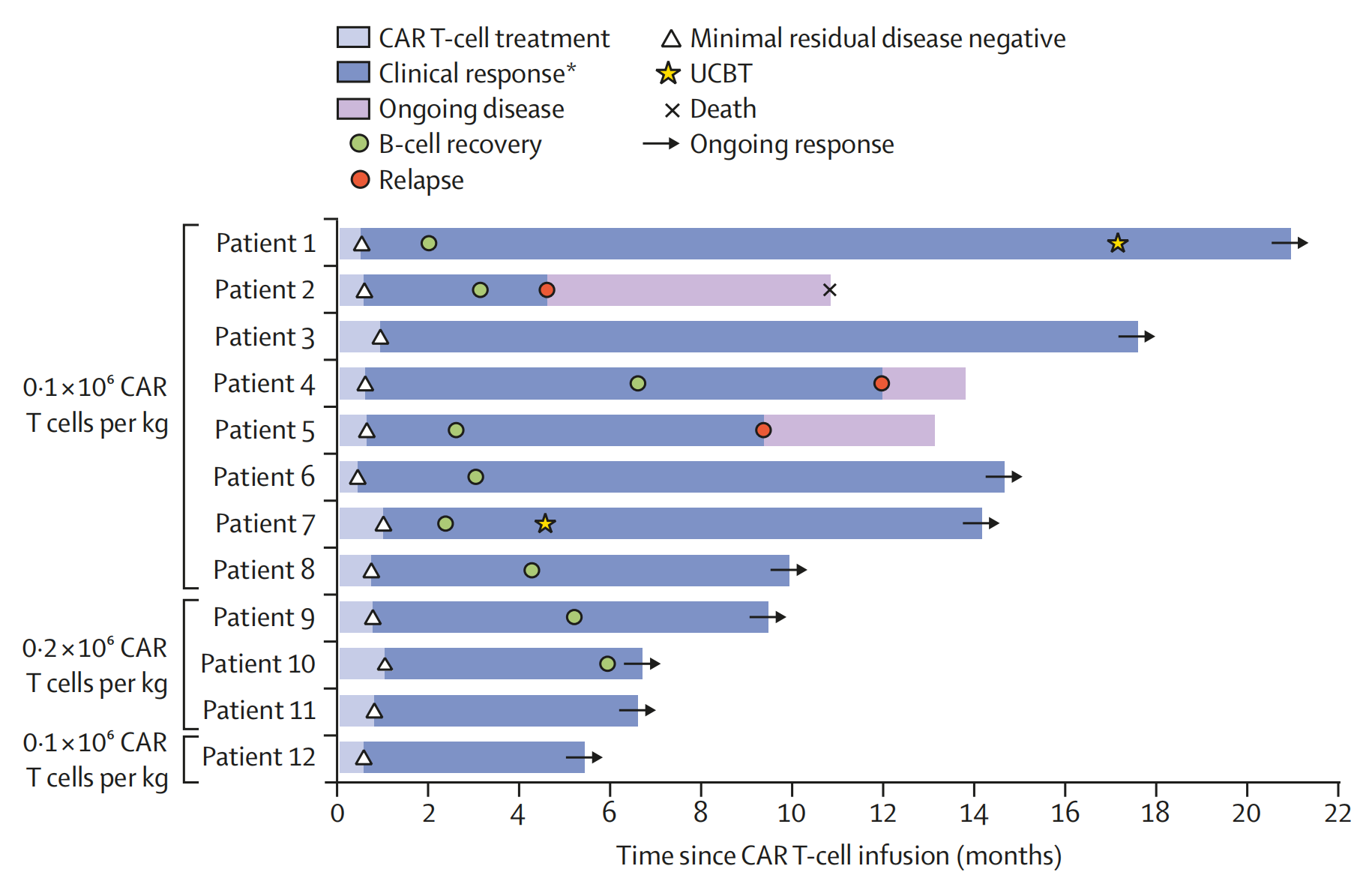
The study enrolled 12 patients with relapsed/refractory B-ALL, with a median follow-up time of 12.5 months. Patients received a single intravenous infusion of Meta10-19 CAR-T cells at doses ranging from 0.1 to 0.2 × 10⁶ cells/kg.
Key Efficacy Results:
At one month post-treatment, the overall response rate (ORR) was 100%. This included 9 patients (75%) achieving complete remission (CR) or CR with partial/incomplete hematologic recovery (CRi), and 3 patients (25%) achieving morphologic leukemia-free state.
By three months, all patients (100%) achieved minimal residual disease (MRD) –negative CR.
Median relapse-free (RFS) and overall survival (OS) had not been reached at the time of reporting. The 6-month RFS and OS rates were as high as 91% and 100%, respectively.
Safety Profile:
The most common Grade 3 or higher adverse events were hematologic toxicities.
Critically, no Grade 3 or higher cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) of any grade was observed.
Pharmacological and Cellular Kinetics:
Meta10-19 demonstrated robust in vivo expansion, with a median onset of expansion on day 8 and peak expansion on day 10 post-infusion.
The geometric mean of the area under the curve (AUC0-28d) for CAR copies was more than six times higher than that of tisagenlecleucel, the first FDA-approved CAR-T products.
Serum IL-10 levels showed a significant positive correlation with CAR-T expansion metrics, confirming the role of IL-10 in enhancing CAR-T cell functionality.
These clinical results demonstrate that IL-10 CAR-T, as a revolutionary approach, not only offers new hope for patients with relapsed/refractory B-ALL but also holds substantial potential for treating other malignancies. The study validates IL-10-mediated metabolic enhancement as a key strategy for overcoming T cell exhaustion. With its superior expansion capacity and finely tuned cytokine regulation, this therapy successfully addresses critical bottlenecks in current CAR-T technologies, setting a new benchmark for next-generation cellular immunotherapies.
About B-ALL and the Meta10 Platform
B-ALL is a malignant clonal proliferative disease originating from B-lineage lymphoblastoid progenitor cells in the bone marrow. It is the most common childhood malignancy, with lower incidence but worse prognosis in adults. Patients who fail frontline therapy or relapse after remission face significantly greater treatment challenges.
CAR-T cell therapy has achieved remarkable success in relapsed/refractory B-ALL. However, approximately 10–30% of patients do not respond, and over 50% of those who do respond eventually relapse. T-cell exhaustion is a major contributor to these limitations.
Previous research from Professor Li Tang’s lab at EPFL identified that IL-10 can enhance oxidative phosphorylation in terminally exhausted T cells, enabling metabolic reprogramming to counteract exhaustion. This discovery led to the founding of Leman Biotech and the development of the proprietary Meta10 platform, which is designed to strengthen immune cell persistence and anti-tumor efficacy.
Building on this novel IL-10–mediated metabolic regulation mechanism, the team developed the metabolically armored IL-10–secreting CAR-T cells (META 10-19). This therapy protects CAR-T cell mitochondrial integrity within the tumor microenvironment, granting it robust resistance to exhaustion. It has demonstrated exceptional curative potential across multiple preclinical models, including subcutaneous tumors, lung cancer metastases, and NSG mouse models with human CD19-positive tumors. The therapy also promotes the formation of stem-like memory T cells, which help prevent tumor relapse. These foundational findings were published in Nature Biotechnology in 2024 under the title “Metabolically enhanced CAR-T cells for cancer immunotherapy.”
With Meta10-19 now achieving outstanding results in clinical trials, Leman Biotech continues to advance its mission of developing metabolic immunotherapies that redefine the boundaries of cancer treatment.