

For many years, immunotherapies such as CAR-T cell therapy and immune checkpoint inhibitors have primarily focused on activating type-1 immunity to eliminate cancer cells. In contrast, type-2 immunity, typically known for responding to parasitic infections, has traditionally been viewed as antagonistic to type-1 immunity and, consequently, as a promoter of tumor progression.
On September 25, 2024, Nature published two studies that reached mutually supportive and consistent conclusions: Type-2 immunity may play a crucial role in maintaining long-term anti-cancer immune responses. This discovery highlights a new and previously underappreciated function of type-2 immunity in cancer, offering the potential to reshape the field of cancer immunotherapy and shift the paradigm for next-generation treatment strategies.
These two studies were co-led by Professor Li Tang’s team from the École Polytechnique Fédérale de Lausanne (EPFL) and Professor Rong Fan’s team from Yale University. Dr. Bing Feng from EPFL and Dr. Zhiliang Bai from Yale University are the co-first authors of both papers. Notably, CAR-T therapy pioneer Professor Carl H. June from the University of Pennsylvania, Professor Stephan A. Grupp from the Children's Hospital of Philadelphia, Professor J. Joseph Melenhorst from the Cleveland Clinic, and Leman Biotech co-founder, Professor Yugang Guo from Zhejiang University, served as co-corresponding authors on the studies.
 In the first study, Professor Li Tang’s team, in collaboration with Yale University and the University of Pennsylvania, analyzed CAR-T cells from the world’s first two cohorts of pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients treated with CAR-T therapy, who have been followed for over 10 years. The research revealed a crucial role of type-2 immune signatures in CAR-T cells, which were instrumental in sustaining more than eight years of cancer-free clinical responses in B-ALL patients.
In the first study, Professor Li Tang’s team, in collaboration with Yale University and the University of Pennsylvania, analyzed CAR-T cells from the world’s first two cohorts of pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients treated with CAR-T therapy, who have been followed for over 10 years. The research revealed a crucial role of type-2 immune signatures in CAR-T cells, which were instrumental in sustaining more than eight years of cancer-free clinical responses in B-ALL patients.
One notable case is Ms. Emily Whitehead, who at seven years old, participated in the first pediatric clinical trial for CAR-T cell immunotherapy in April 2012 at the Children’s Hospital of Philadelphia. Emily, who had recurrent acute lymphoblastic leukemia (ALL), has been in remission for 12 years and is now considered cancer-free. However, this kind of long-term success is rare. In most cases, over half of B-ALL patients relapse within a year following CAR-T therapy.
To unravel the mystery behind achieving long-term remission and investigate whether CAR-T cells from patients like Emily possess distinct characteristics or markers that differentiate them from those in relapsed patients, the research team collected over one million pre-infusion CAR-T cells from 82 pediatric B-ALL patients and six healthy donors. Using single-cell multi-omics sequencing, they precisely mapped the connection between CAR-T cell functionality and the persistence of clinical response.
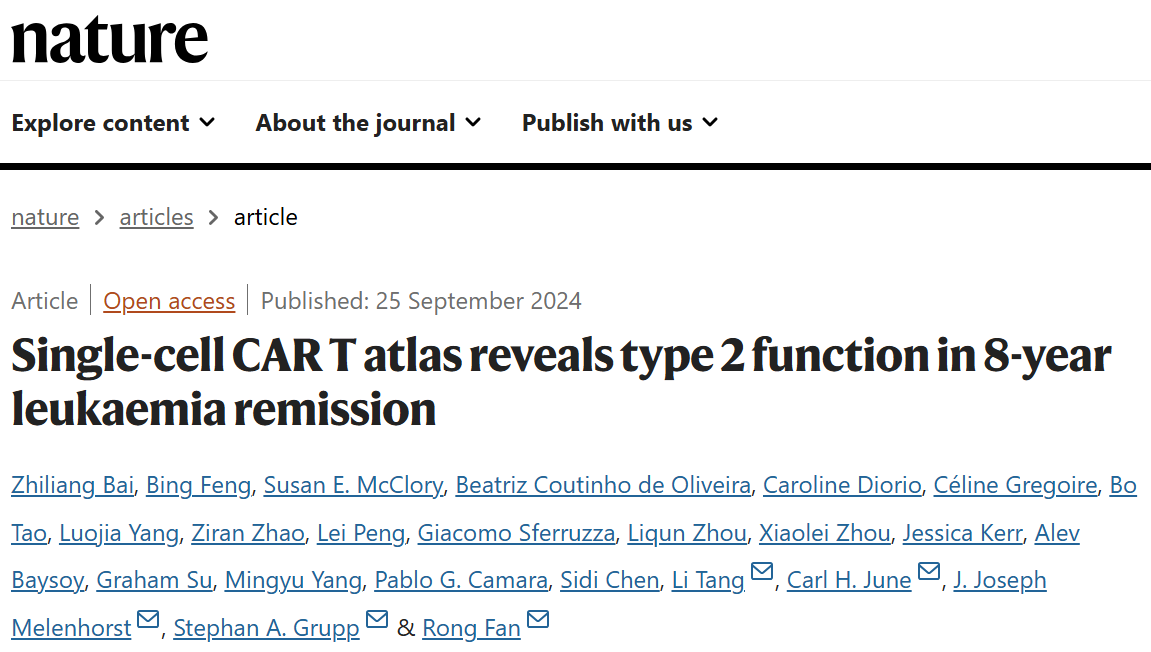
In clinical practice, patient B-cell aplasia (BCA) is often employes as a surrogate marker for the durability of CAR-T efficacy. The study results showed that single-cell clustering analysis found a significantly higher proportion of type-2 CAR-T cells in both the BCA-L group (which included five patients with a median remission duration of over 8.4 years) and the BCA-O group (which included 11 patients with a median remission duration of over 5 years) compared to other patients. To further examine the role of type-2 immunity following CAR-T cell reinfusion, the team conducted proteomic analyses on 345 serum samples collected from 33 patients at different intervals over a nearly two-month period post-CAR-T treatment. The results confirmed that significantly elevated levels of type-2 cytokines were present in the serum of patients in both the BCA-L and BCA-O groups (Figure 1). These findings prompted the researchers to validate the results using animal models. In these models, type-2 high CAR-T cells exhibited more than tenfold enhanced proliferation compared to type-2 low CAR-T cells. Additionally, type-2 high CAR-T cells demonstrated stronger memory properties and reduced exhaustion markers, which significantly extended the durability of the anti-tumor effects, particularly following secondary rechallenge by tumor cells.
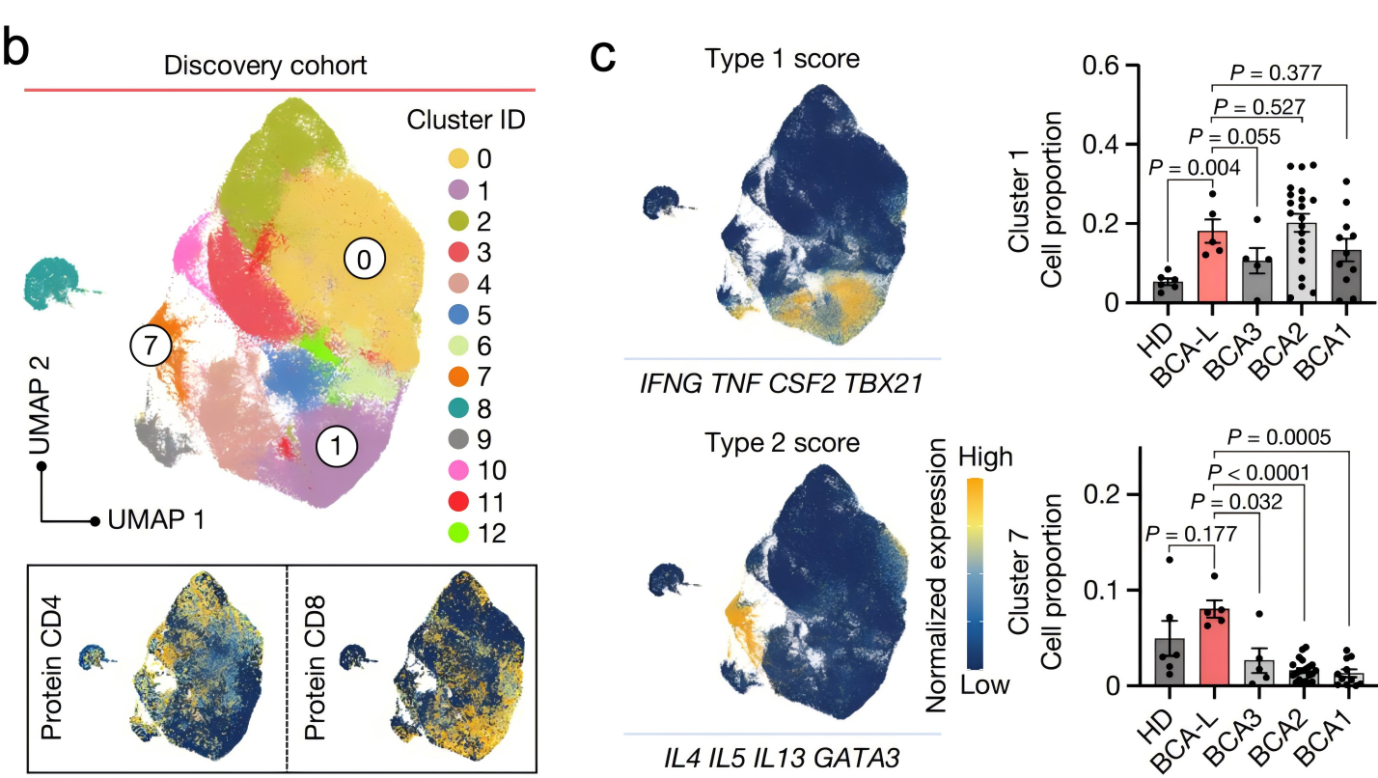 Figure 1. Large-scale single-cell multiomics sequencing reveals the critical role of type-2 CAR-T in maintaining an 8-year ultralong remission
Figure 1. Large-scale single-cell multiomics sequencing reveals the critical role of type-2 CAR-T in maintaining an 8-year ultralong remission
To further investigate the role of type-2 immunity following CAR-T infusion, the research team conducted proteomics analysis on 345 serum samples collected at various time points from 33 patients during the first two months after CAR-T treatment The analysis confirmed significantly elevated levels of type-2 cytokines in the sera of patients in the BCA-L and BCA-O groups (Figure 1). Based on these findings, the researchers validated the results in animal models. The results showed that type-2 High CAR-T cells had more than a tenfold increase in proliferation compared to type-2 Low CAR-T cells. Additionally, they demonstrated enhanced memory characteristics, reduced exhaustion, and significantly improved the durability of anti-tumor effects, particularly following secondary rechallenge by tumor cells.
To further explore the relationship between type-2 immunity and long-term cancer remission, in the second study, Professor Li Tang's team and Professor Rong Fan's team focused on using a type-2 immune cytokine with a longer half-life, Fc–IL-4. By combining Fc-IL-4 with CAR-T cell therapy and immune checkpoint inhibitor therapy (Type-1 centric immunotherapy), they investigated its impact on solid tumors. The study revealed that type-2 immune cytokines, when combined with type-1 centric immunotherapy, exhibited strong anti-tumor effects in various syngeneic and xenograft solid tumor models and activated long-lasting immune memory responces.
For example, in the YUMM1.7-OVA melanoma model, the combination of Fc-IL-4 and OT1 cell therapy achieved 100% tumor clearance. In the MC38-HER2 colon cancer model, Fc-IL-4 combined with HER2-CAR-T cell therapy achieved 87% tumor clearance. In the Raji model of human lymphoma in NSG mice, huFc-IL-4 combined with CD19-CAR-T cell therapy cleared 75% of tumors (Figure 2). Furthermore, in the MC38 colon cancer model, Fc-IL-4 combined with immune checkpoint inhibitors achieved 100% tumor clearance. All mice with cleared tumors were able to resist subsequent rechallenge of tumor cells, ultimately achieving long-term immune protection.
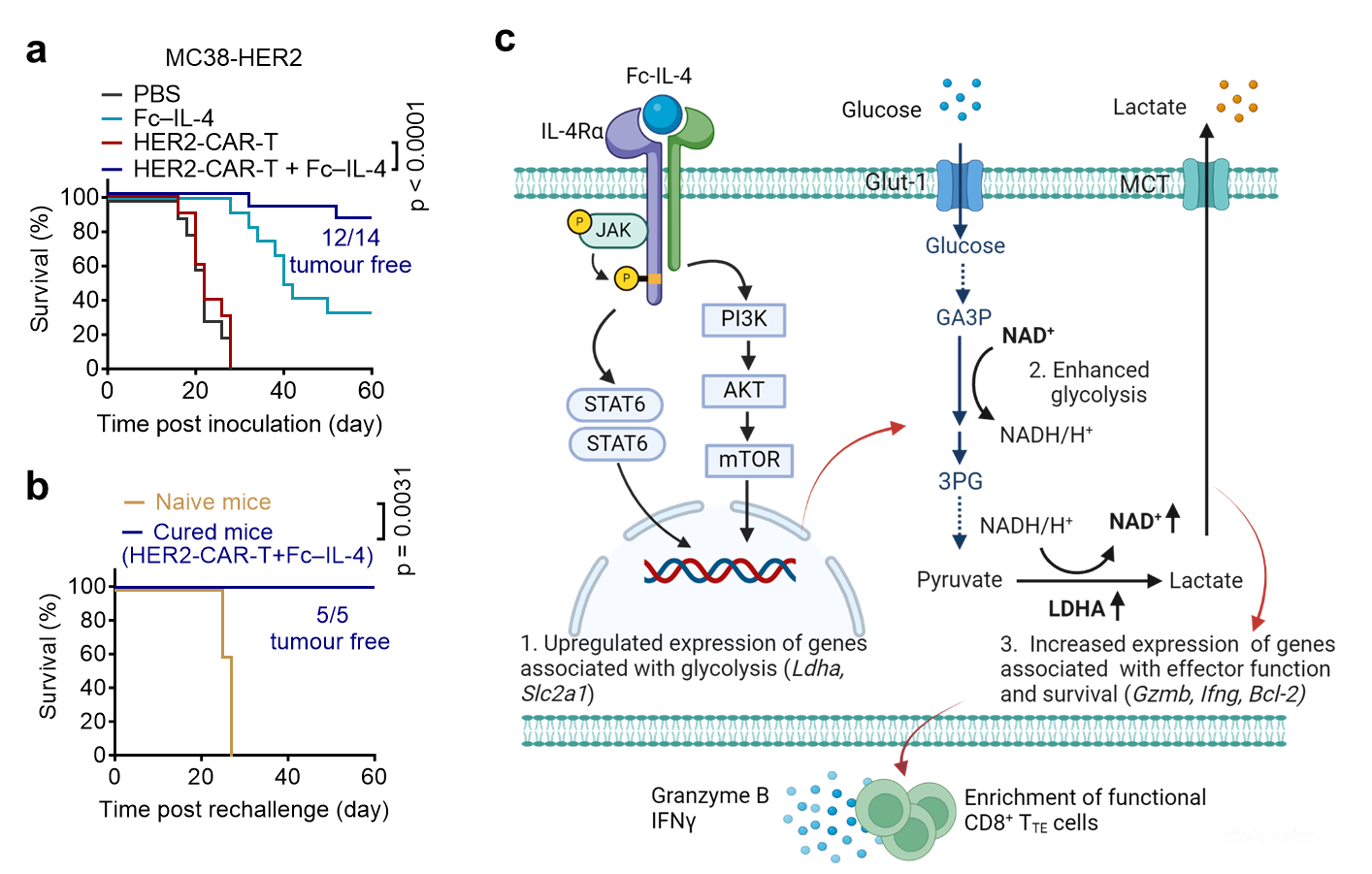 Figure 2. Type-2 immune cytokine Fc-IL-4 cooperates with type-1 immunotherapy to effectively clear solid tumors
Figure 2. Type-2 immune cytokine Fc-IL-4 cooperates with type-1 immunotherapy to effectively clear solid tumors
The researchers also investigated the immunological and molecular biological mechanisms of Fc-IL-4 in anti-tumor activity.They found that the efficacy of Fc-IL-4 in tumor suppression is due to its direct action on terminally exhausted CD8+ T cells within tumors. Fc-IL-4 significantly reduced T cell apoptosis, increased the number of terminally exhausted CD8+ T cells, and enhanced their capacity to destroy tumor cells. As a typical type-2 cytokine, Fc-IL-4 worked synergistically with type-1 immunotherapy to trigger a sustained anti-cancer response.
This study not only uncovered the synergistic anti-tumor effects between type-1 and type-2 immune responses but also innovatively used type-2 immune factors to demonstrate their potent anti-cancer effects in preclinical animal models. This discovery provides a new paradigm for the development of next-generation cancer immunotherapies, promoting further breakthroughs in tumor immunotherapy by effectively orchestrating type-1 and type-2 immune responses.
Find the full publication here:
https://www.nature.com/articles/s41586-024-07762-w
https://www.nature.com/articles/s41586-024-07962-4
About Leman Biotech
Leman Biotech is a clinical-stage cancer immunotherapy company co-founded by Professor Li Tang of the Swiss Federal Institute of Technology in Lausanne (EPFL) and AI-driven drug R&D leader XtalPi. The company combines innovative metabolic reprogramming technology with a cutting-edge AI drug discovery platform to develop next-generation cancer immunotherapies. The core technology, Meta 10, has the potential to cure solid tumors, as demonstrated in published research in top academic journals such as Nature Immunology and Nature Biotechnology. Additionally, it holds PCT patents covering major economies worldwide. Leman Biotech completed a successful angel plus round of financing, raising nearly $18 million in total, with plans for further financing underway. The company is committed to finding solutions to tackling the bottlenecks in cancer immunotherapy, improving response rates and efficacy, and making unremitting efforts to achieve the ultimate goal of curing cancer.